Table of Contents
- Online resources and software environment
- Simple sampling of RNA sequences
- Adding complementarity constraints from an RNA secondary structure
- Controlling the GC content
- Controlling the BP energy
- Further constraints
- Targeting specific GC content and energy
- Targeting Turner energy
- Add IUPAC constraints
Online resources and software environment
This document is hosted online as Jupyter notebook with precomputed results. Download this file to view, edit and run it in Jupyter.
We recommend to install all required software using Mamba (or Conda) and PIP.
Start the Jupyter notebook server after activating the environment
The original sources are part of the Infrared distribution and hosted on Gitlab (in Jupytext light Script format).
Simple sampling of RNA sequences
We start by importing the infrared module (and assign a shortname).
Let's specify our first constraint network model. It is very simple: we define 20 variables, each with 4 possible values, which encode a sequence of nucleotides. Our first model has no dependencies.
From this model, we directly construct a sampler.
Once initialized with a model, the sampler can prepare the sampling from this model. In particular, it computes a tree decomposition of the constraint network. In general, it is interesting to inspect the tree decomposition of a model's constraint network, since it guides the computation.
We define a function to display information on the tree decomposition.
When we now call this function on our sampler, we will see that so far our model has a trivial tree decomposition (as expected, since we didn't add any constraints or functions to our model).
tree width = 0 bags = [[5], [14], [10], [1], [6], [12], [16], [18], [9], [0], [3], [8], [2], [7], [15], [19], [17], [4], [11], [13]] edges = [(0, 1), (0, 2), (0, 3), (0, 4), (0, 5), (0, 6), (0, 7), (0, 8), (0, 9), (0, 10), (0, 11), (0, 12), (0, 13), (0, 14), (0, 15), (0, 16), (0, 17), (0, 18), (0, 19)]

Nevertheless, we can evaluate the cluster tree.
In this simple case, this will count the nucleotide sequences of length 20 (i.e. we expect to see a count of 4**20).
# = 1099511627776
Next, let's draw 10 samples. These samples encode uniformly drawn sequences of length 20.
[[0, 0, 0, 0, 2, 1, 0, 2, 2, 0, 3, 1, 1, 2, 0, 2, 0, 3, 3, 3], [3, 3, 1, 2, 1, 2, 3, 3, 3, 1, 0, 2, 1, 3, 3, 1, 1, 2, 3, 2], [3, 3, 1, 0, 2, 2, 1, 0, 0, 3, 2, 0, 2, 0, 2, 0, 1, 3, 3, 1], [0, 3, 2, 2, 3, 2, 0, 2, 1, 1, 1, 1, 1, 0, 2, 1, 3, 3, 3, 2], [0, 1, 2, 1, 3, 3, 2, 3, 3, 3, 2, 2, 3, 2, 1, 1, 0, 1, 3, 3], [0, 1, 0, 0, 0, 3, 2, 1, 3, 1, 2, 1, 3, 1, 1, 2, 3, 0, 3, 1], [3, 2, 0, 0, 0, 1, 3, 3, 2, 0, 2, 2, 0, 0, 3, 0, 0, 0, 2, 2], [3, 0, 1, 2, 0, 2, 3, 0, 3, 0, 3, 0, 0, 3, 0, 0, 3, 2, 0, 0], [2, 3, 3, 1, 3, 3, 2, 1, 3, 2, 1, 0, 0, 0, 3, 0, 2, 2, 1, 0], [2, 3, 3, 3, 2, 2, 0, 3, 3, 3, 3, 2, 1, 3, 0, 3, 2, 0, 3, 2]]
... and show them more pretty.
['AAAAGCAGGAUCCGAGAUUU', 'UUCGCGUUUCAGCUUCCGUG', 'UUCAGGCAAUGAGAGACUUC', 'AUGGUGAGCCCCCAGCUUUG', 'ACGCUUGUUUGGUGCCACUU', 'ACAAAUGCUCGCUCCGUAUC', 'UGAAACUUGAGGAAUAAAGG', 'UACGAGUAUAUAAUAAUGAA', 'GUUCUUGCUGCAAAUAGGCA', 'GUUUGGAUUUUGCUAUGAUG']
Adding complementarity constraints from an RNA secondary structure
We define a toy RNA secondary structure (in dot-bracket notation)
... and parse it.
[(0, 10), (1, 9), (2, 8), (3, 7), (11, 19), (12, 18), (13, 17)]
Next, we define the class of complementarity constraints; we call it BPComp for base pair complementarity. Each such constraint is constructed for two specific positions i and j. Then, the constraint is checked by testing whether the values x and y at the respective positions i and j correspond to complementary base pairs.
Btw, there is already a pre-defined constraint rna.BPComp, which we could have used as well.
From the parsed structure, we generate a set of complementarity constraints - one for each base pair.
For illustrations, let's also print the variables of the constraints.
[[0, 10], [1, 9], [2, 8], [3, 7], [11, 19], [12, 18], [13, 17]]
Now, we are ready to construct the new constraint model, including the new constraints, and construct the corresponding sampler.
Let's first see whether the tree decomposition looks more interesting this time.
tree width = 1 bags = [[19, 11], [9], [1, 9], [8], [8, 2], [10], [0, 10], [18], [12, 18], [13], [17, 13], [7], [3, 7], [5], [15], [4], [16], [14], [6], [19]] edges = [(0, 1), (0, 3), (0, 5), (0, 7), (0, 9), (0, 11), (0, 13), (0, 14), (0, 15), (0, 16), (0, 17), (0, 18), (1, 2), (3, 4), (5, 6), (7, 8), (9, 10), (11, 12), [19, 0]]
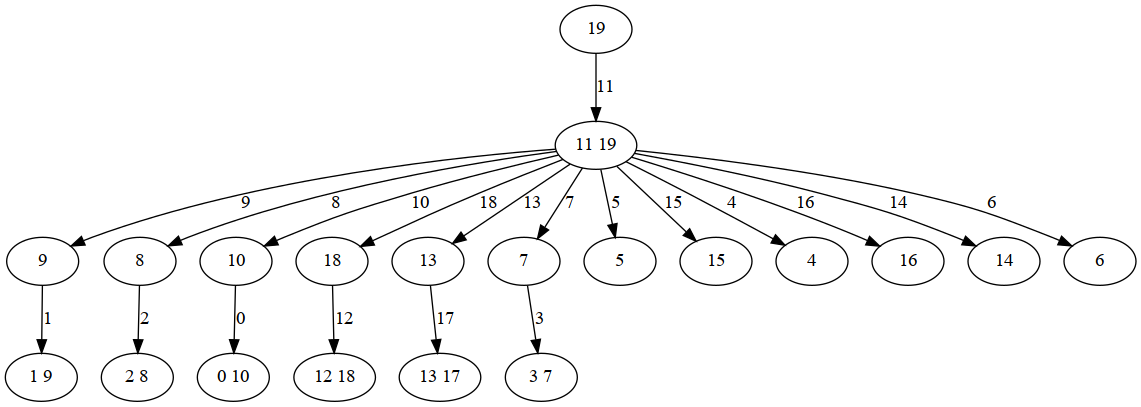
As expected, the tree decomposition reflects the dependencies due to the new constraints.
When we evaluate the model, we obtain the number of sequences that are compatbile with our secondary structure (doing simple combinatorics, we expect \(6^7 * 6^4\)).
# = 1146617856
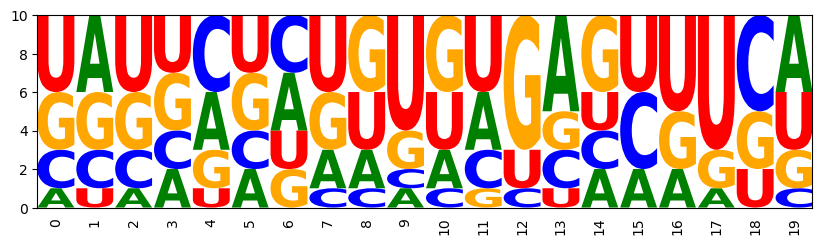
It's time to generate samples again. Before this get's boring, we quickly define a function to automatize this. For the fun of it, let's also draw sequence logos from the samples.
Note: logos will be drawn only if the modules logomaker and matplotlib.pyplot are properly installed.
Generate samples and print them with the given structure; we can see that the base pair constraints are satisfied by all samples.
((((...))))(((...))) GUAUCAGAUGCCUCACGGGG AUCAAGCUGGUACUUCCAGU CAGAGCCUCUGGCUAGGGGU GGGGCCGCUUCUAUGUGAUA UAUGUACUAUGUGCUUUGUA CGUCGACGACGUGUCGAGCG CUGCCGUGCGGGUAUCCUAU GAGCAGGGUUCCAGUUCCUG GUAGUACUUACAGAACUUUU CCGAAUUUCGGGUAACAUGC UCUAUAGUGGGUAGGAACUG GCGUAGGACGCGGUUACGCC CUUUGGCGGGGCAUCCCGUG UUGUGACACGACGCGAUGUG UGGUCAGAUUGGAUUUGGUC GGUGGUGUGUUGAGUUCUUC CGGUUGCGUUGGCGAUUUGC UUCUCCGAGGGUUCAGUGAA GGUAAUGUGCUUGGGGAUUA UCCACUUUGGGAGUUUAGCU Matplotlib is building the font cache; this may take a moment.

Controlling the GC content
To control features of our samples like the GC content, we are going to add suitable functions to our model. For the GC control, we define the function class GCCont, which let us count the Gs and Cs in the encoded sequence. For a specific position i, the function returns 1 if the encoded nucleotide is G or C; otherwise, 0.
Btw, this function is as well pre-defined in Infrared's rna support module as rna.GCCont.
GC-control is added to the sampler, simply by specifying the set of functions (gc_funs), adding them to the sampler as a function group ‘'gc’`, and setting the weight of the corresponding (automatically generated) feature of the same name.
Note how the new functions can simply be added to the existing model with base pair complementarity constraints. This provides a first good example of the compositional construction of models in Infrared.
((((...))))(((...))) UGGGAACUCUAAGUGUAAUU CCUGCUCUGGGUGAUAUUUA UACUCGAGGUGUGAUAAUCA UAGGCCCCUUACGGCCUUCG CUUUAUUAAAGAUAGCUUGU UGUCUGAGGCGUGCAUUGCA GGUCCGAGAUCCGCAUGGCG GAGAGUGUUUUGCGGCUUGC GCAAGAUUUGUAGACCGUCU AACUACGAGUUUUAGUGUGA
Controlling the BP energy
Next, we are going to introduce functions to compute the energy our secondary structure for the enoded sequence. To express energy in the base pair energy model, we are going to define a set of functions of the pre-defined type rna.BPEnergy; one for each base pair.
These functions are added to the existing model, as the GC functions before. We put them into a new function group ‘'energy’, which will immediately allow us to control them (in addition to the GC content) through the automatically generated feature'energy'`.
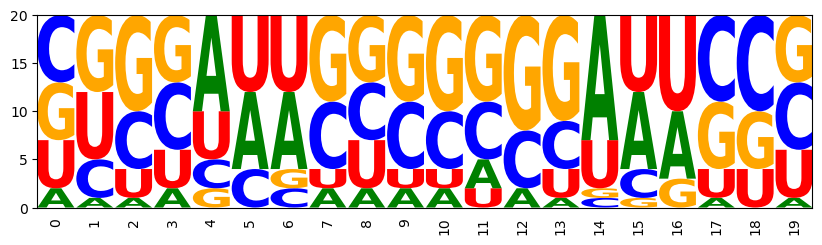
Let's set weights of our two features and generate samples. By setting a negative weight for energy, we aim at low energy for the structure. The GC control allows us to counter-act the induced shift towards high GC content. For the latter purpose, we set a negative weight to aim at lower GC content.
((((...))))(((...))) CGAGUUCCUCGAGGAUUCCU CCCACGUUGGGAGUGAUACU CUGGUUACUAGUUCGAUGAG CAGUCUUGCUGGUCCCAGGC AGGCAAUGCUUGCGAUUCGU GGUUAUGAACUUGCUGAGCG CGUGAUCCGCGGCCACCGGC GCUCUUCGAGCUCCCCGGGA UGCCAAGGGCAGUCUGUGGU CGGCUCGGCUGGGGACACCC

Further constraints
We can even extend the model by further constraints, while maintaining the ability to control the features. As example of additional hard constraints, we are going to avoid GG dinucleotides. Extending the model by such additional constraints, expectedly, goes through the steps of
- defining the new constraint type
- defining a set of constraints
- adding the set to the model
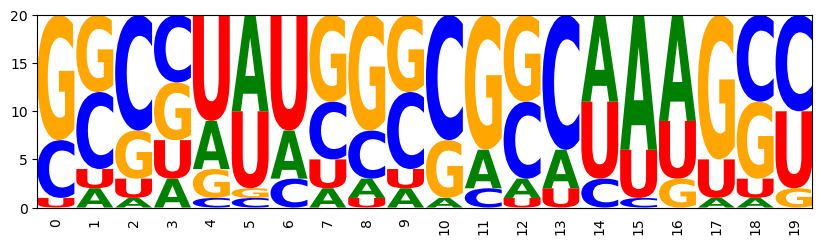
Finally, we construct the sampler for the extended model and generate samples.
((((...))))(((...))) AGCGUGUCGUUAGCGAAGCU AGUUAAAAGCUUAUGCCAUA CGACUAUGUCGUGAAUAUCA GAAAAGAUUUUUUGUCCCAA CAAUAUAAUUGUGUUUUACG UUAGAAUCUAGUAAUACUUA GUCGUGCCGACUAAUCGUUA GAGACAAUCUCGAUGAUGUU UGAGCACUUCAGUGAAUCAC UACUCCCAGUGCGAAUGUCG

Targeting specific GC content and energy
The previous model allowed to control the mean GC content and base pair energy of the produced model by manually tweaking the weights.
This leaves several challenges if we would want to produce samples with defined GC content and energy:
- we would have to filter the generated samples for a specific tolerance range around the targeted values
- the features are not independent, thus changing one weight requires changing the other - this would get even harder for more than two features.
Infrared supports these goals in a general way by implementing a multi-dimensional Boltzmann sampling strategy.
For this purpose, first set up the model and sampler as before.
Infrared now allows to set target values and tolerances for all (or selected) features and then produce targeted samples using the method Sampler.targeted_sample().
In the code below, we print samples with their energy and GC-content. Note how we obtain these values simply by asking the model to evaluate the features for the sample.
CCGUACAGCGGUCGUAACGA -11.92 12.00 GGGGCUUCUCCCGGAUUUUG -11.06 12.00 GGCGCAACGCCAGAUUGUCU -11.56 12.00 UGGGAUUCUCGGGGAAGUCC -11.06 12.00 CGCCUCAGGCGGGUAAUGUU -11.06 12.00 CCCCUUCGGGGAACAUAGUU -11.56 11.00 GUUCAAGGGGCGGUAUUGCC -11.06 12.00 UUGCAAAGUGGGGGACACCC -11.06 12.00 UUGCACAGCGACGCAAAGCG -11.92 12.00 CUGCGUUGUGGUGGAUUCCG -11.06 12.00 GGAUAAAAUCCCCCAAUGGG -11.56 10.00 CCUAUAUUAGGCCGCAACGG -11.56 11.00 UCCCAAUGGGAACGACUCGU -11.56 11.00 UUGGCUUCCAGGCCAAUGGC -11.92 12.00 CGUGAAUCACGGGUUGUACC -11.56 11.00 AGGUGUAACCUGCGUUGCGC -11.56 12.00 GUGGUUACCACCACGCUGUG -11.56 12.00 AGCGAAGCGUUCGGAUUCCG -11.92 12.00 CUCUUCUGGGGGGGUUACCU -11.06 12.00 GAGAAUCUCUCGGGAAACCC -11.56 11.00
Targeting Turner energy
Finally, for real applications, it is typically much more relevant to target specific Turner energy than specific energy in the simple base pair model. In Infrared, we solve this by defining and adding a new feature ‘'Energy’to the model that calculates Turner energy (by calling a function of the Vienna RNA package). In addition to defining this calculation, we specify as well that the new feature should control the function groupenergy`. In this way, Infrared will use base pair energy as proxy of Turner energy.
Note: this requires the Vienna RNA package with working Python bindings (currently, this fails in Windows even after installing the package from binaries)
As before, we set targets and generate samples. Of course, this time, we set a target range for the new feature of Turner energy.
CCGCUAAGCGGACCUUAGGU -13.14 -4.80 12.00 GGUGAUCCACCGGACAAUCC -11.56 -4.00 12.00 GUCUGAUGGACGGCUAUGCC -11.92 -4.10 12.00 GACUUAUGGUCGCCUUGGGC -11.92 -4.10 12.00 GGCCUUUGGCCGACCUUGUU -11.92 -4.20 12.00 CCCAGAUUGGGGGCUAAGCU -11.92 -4.10 12.00 GGCAUUUUGCCGCCAUGGGU -11.92 -4.80 12.00 GGCUUUCAGCCGCUAAAGGC -11.92 -4.50 12.00 GAGGUCUCCUCAGCUAUGCU -11.56 -4.00 11.00 CCCCAUUGGGGGGAAAAUCC -13.14 -5.60 12.00 CCCCUAUGGGGCACAAAGUG -13.14 -4.00 12.00 UGCCAAUGGCACGCUAUGCG -13.14 -4.30 12.00 GCCCGUUGGGCGGAAAAUCU -11.92 -4.70 12.00 GCGGUAACCGCACCAAAGGU -13.14 -5.30 12.00 GGGUAGAACCCAGCCAUGCU -11.56 -4.50 12.00 CCCGUAACGGGGCUUAUAGC -13.14 -4.50 12.00 GUCAAAAUGACGCCACGGGC -11.56 -4.10 12.00 GGUGUAUCACCGGCAUAGCC -13.14 -5.50 12.00 GGGCCUCGCCCGUAUAAUAC -11.56 -4.20 12.00 CCAGUUUCUGGGCCAAAGGC -13.14 -4.70 12.00
Add IUPAC constraints
In many design applications, we have prior knowledge on the sequences, which can be encoded as IUPAC string. Below, we generate constraints from a IUPAC string and add them to the Infrared model for targeting Turner energy and GC content.
GGGCUUUGCCCCAGUAACUG GGCCUAAGGCCCACUUAGUG GGGCUUAGCCCCGAAUAUCG GGGGUAACCCCCACUAAGUG GCCCUAUGGGCCACAUAGUG GCCCUAAGGGCCGUAUAACG GGGCUUUGCCCCGUAUAACG GGCCUAAGGCCCGUAUAACG GCCCUUAGGGCCAGUAACUG GGGGUAUCCCCCGAAAAUCG GCCCUAUGGGCCGUUUAACG GGGCUUUGCCCCGAAUAUCG GGGGUAUCCCCCGAAUAUCG GGGCUUAGCCCCGAAUAUCG GGGGUAACCCCCGAAAAUCG GGGGUUUCCCCCAGAAACUG GGCCUAUGGCCCACUAAGUG GGGCUAUGCCCCGAUAAUCG GGGCUUUGCCCCACAAAGUG GCCCUUUGGGCCGAAUAUCG
Remark: At this point, we have implemented the full functionality of the RNA design approach IncaRNAtion (Reinharz et al., Bioinformatics 2013) in Infrared. In Infrared, we can easily extend the model further by including further constraints and going on to multi-target design. Infrared makes the latter, which extends functionality to the tool RNARedPrint (Hammer et al; BMC Bioinf 2021), look surprisingly simple. (We demonstrate this in a separate accompanying notebook, as well as in the Infrared-based application RNARedPrint 2).